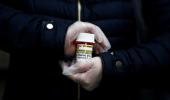
The World Health Organisation on Monday (local time) temporarily suspended the clinical trial of malarial drug hydroxychloroquine for the treatment of coronavirus.

In a briefing, WHO director-general Tedros Adhanom Ghebreyesus said that in view of a paper published last week in the Lancet medical journal, that showed people taking hydroxychloroquine were at higher risk of death and heart problems than those that were not.
There would be 'a temporary pause' on the hydroxychloroquine arm of its global clinical trial, the WHO chief stated.
"The executive group has implemented a temporary pause of the hydroxychloroquine arm within the Solidarity Trial while the safety data is reviewed by the Data Safety Monitoring Board," Ghebreyesus said.
"The other arms of the trial are continuing. This concern relates to the use of hydroxychloroquine and chloroquine in COVID-19. I wish to reiterate that these drugs are accepted as generally safe for use in patients with autoimmune diseases or malaria," he added.
US President Donald Trump has repeatedly touted hydroxychloroquine as a potential game-changer in fighting the coronavirus.
Meanwhile, a study has claimed that hydroxychloroquine and azithromycin, which have been been proposed for treatment of COVID-19 patients, are a potentially lethal combination, and may have a serious impact on the cardiovascular system.
The researchers, including those from Vanderbilt University and Stanford University in the United States, carried out an observational, retrospective meta-analysis of a WHO database encompassing over 21 million adverse event case reports.
The reports span across all medication classes from over 130 countries between November 14, 1967, and March 1, 2020 -- mainly before the COVID-19 pandemic.
The study, published in the journal Circulation, compared cardiovascular adverse-drug-reactions (CV-ADRs) in patients who received hydroxychloroquine, azithromycin, or the combination of both medications with all other cardiovascular medications in the database.
Hydroxychloroquine and azithromycin, alone or in combination, have been proposed for treatment of COVID-19 patients, the researchers said.
They explained that reports of potentially lethal acute cardiac proarrhythmogenic effects -- promoting irregular heart rhythms -- have been described mainly with azithromycin but also with hydroxychloroquine.
Their combination yielded an even stronger signal, the scientists said.
Hydroxychloroquine was also associated with potentially lethal heart failure when exposure was prolonged over several months, according to the researchers.
From the more than 21 million case reports of adverse drug reactions, the researchers extracted case reports for hydroxychloroquine and azithromycin, alone or in combination.
They found that 76,822 adverse event reports were associated with hydroxychloroquine alone, and in 28.4 per cent of those cases (21,808), the drug was suspected to be associated with the adverse event.
The study found that 89,692 adverse event reports were associated with azithromycin alone, and in 60.8 per cent of those cases (54,533), the medication was suspected to be associated with the adverse events.
The researchers said 607 adverse event reports were associated with the combination of both medications.
-- with PTI inputs









 © 2025
© 2025